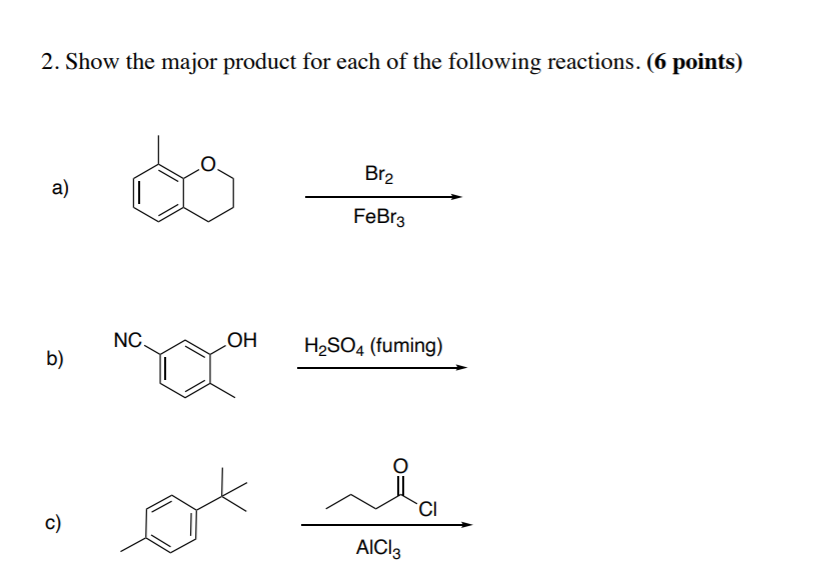
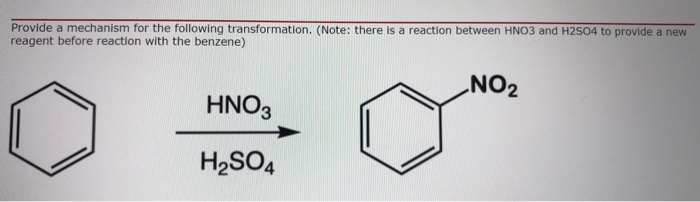
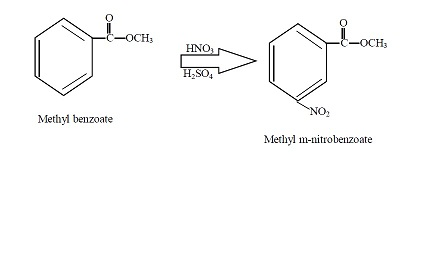
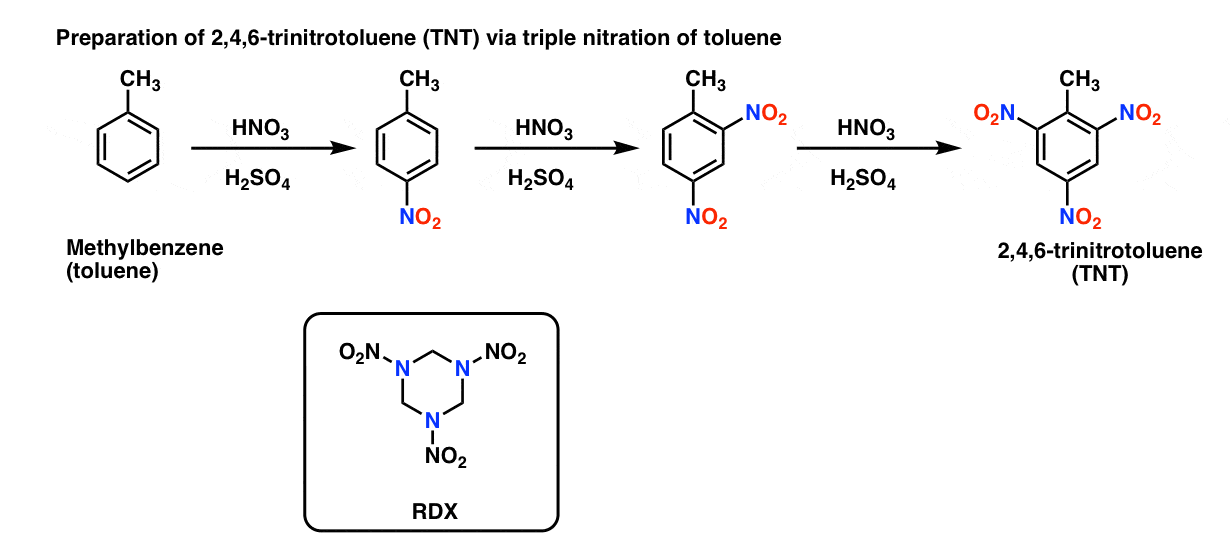
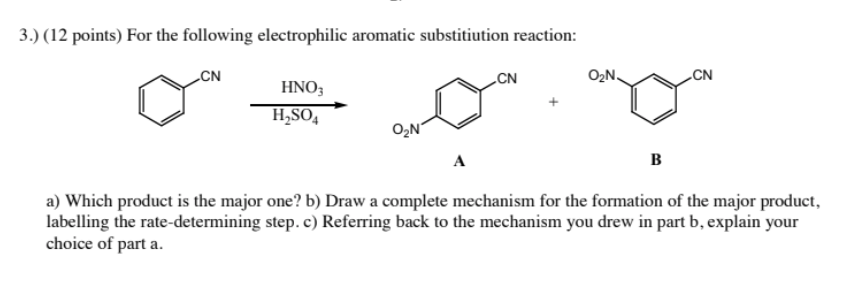
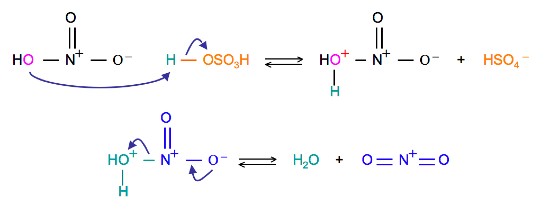
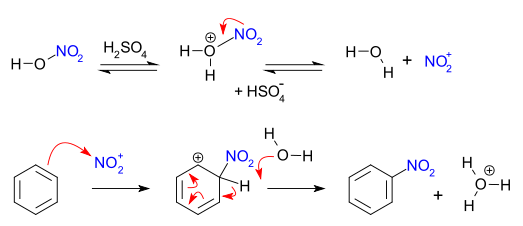
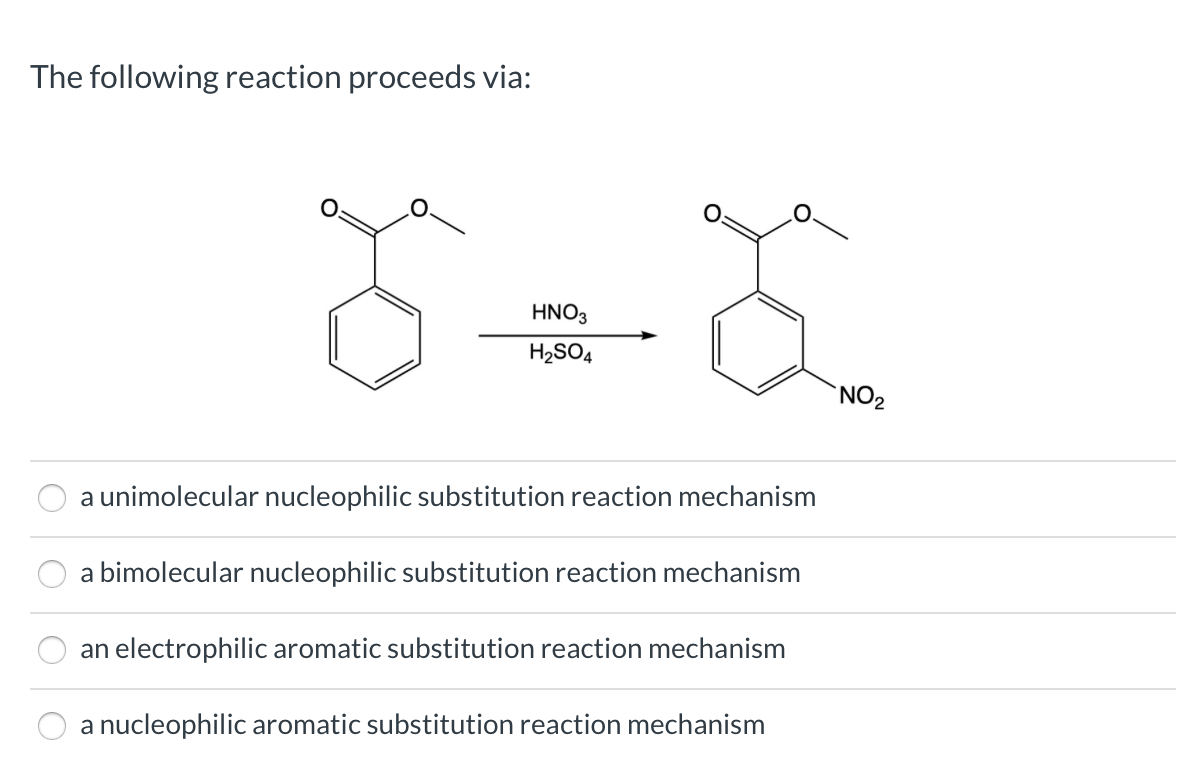
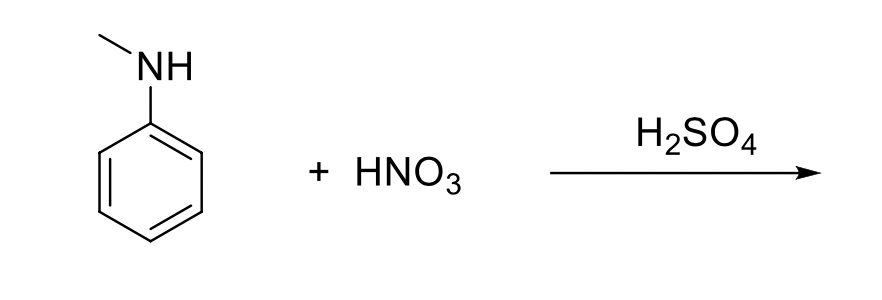reaction mechanism - Nitration of tri-substituted benzene with Acetic acid and HNO3 - Chemistry Stack Exchange

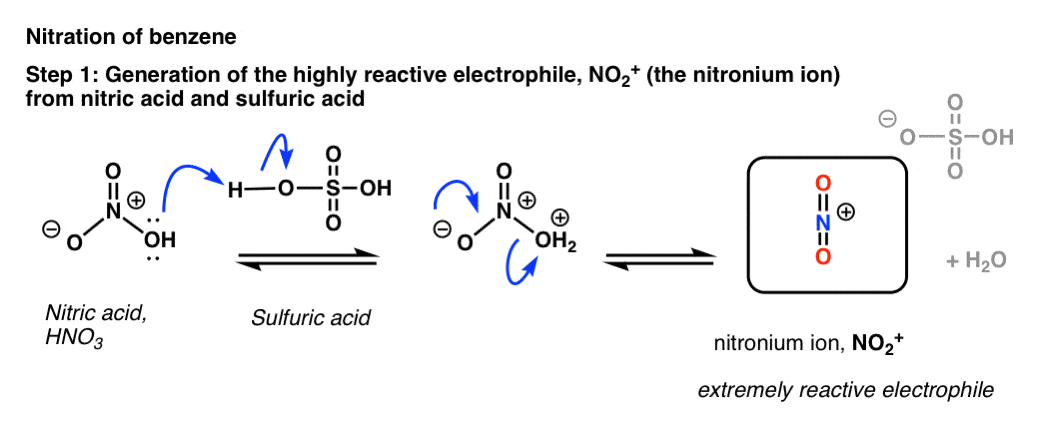
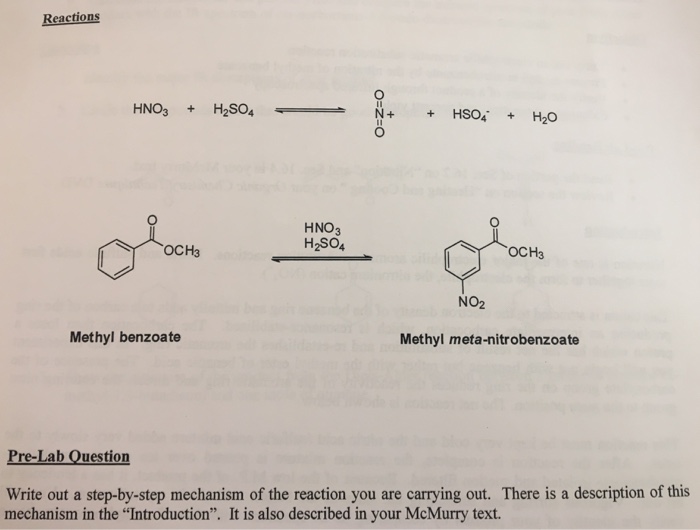
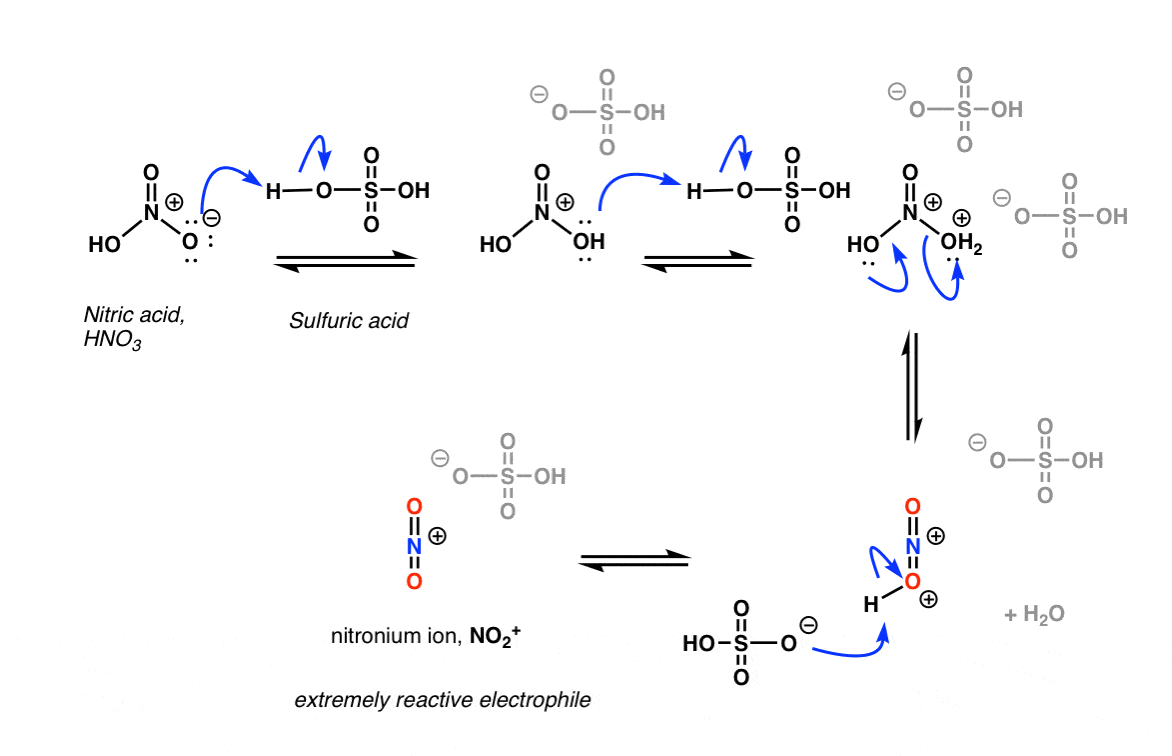
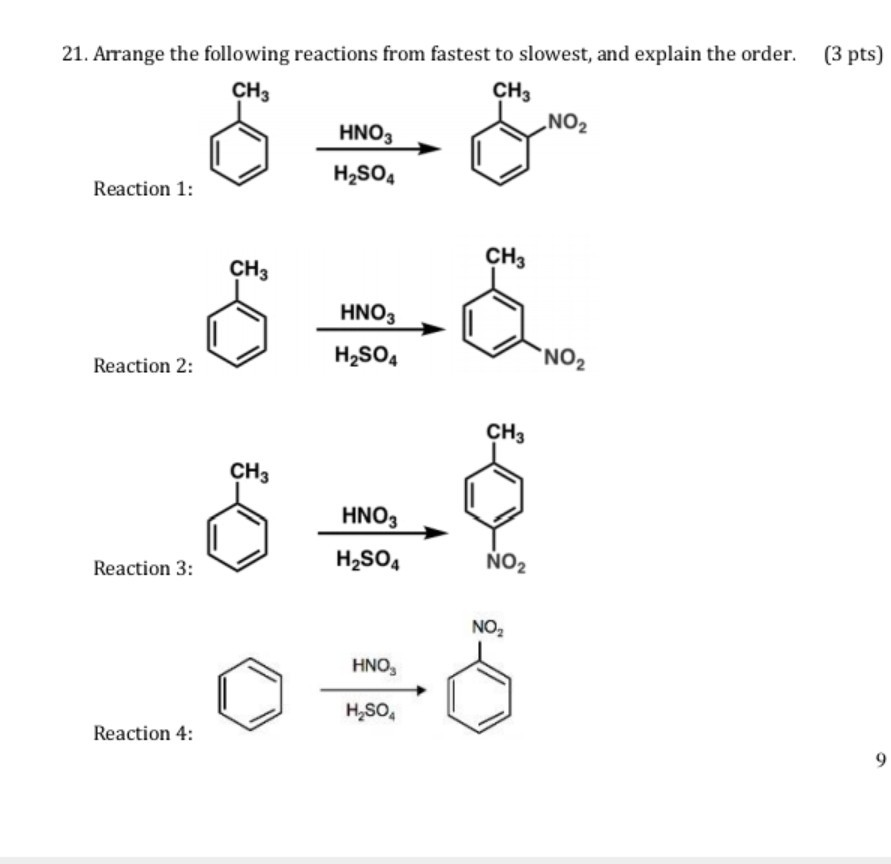
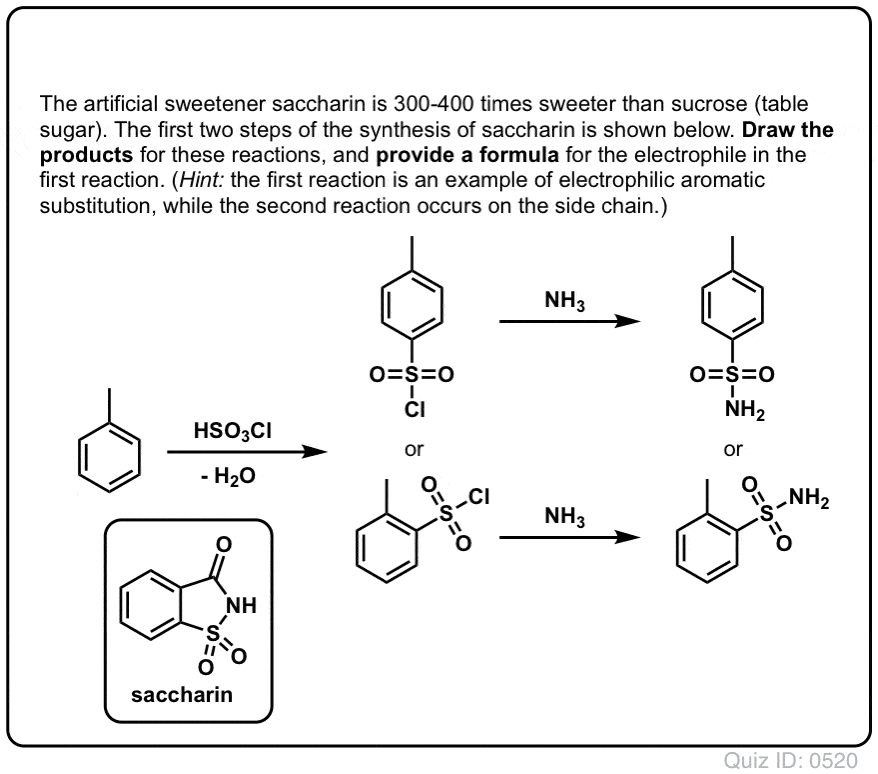
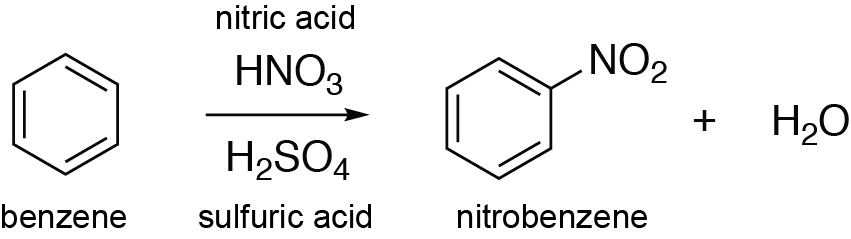
Chemistry lovers - Nitration: A nitro group can be introduced into benzene by using a nitrating mixture to form nitro benzene. The nitrating mixture is a mixture of concentrated nitric acid and

organic chemistry - Reaction of phenol with sulphuric acid and nitric acid - Chemistry Stack Exchange